Material limitation and product-end-of-life issues have become an increasingly interesting topic of discussion for both governments, businesses and societies around the world. In recent years, it has been regarded one of the great environmental liabilities and economic opportunities. One of the newest topics within sustainable business practices has been the adaptation of circular supply chains.
Material limitation and product-end-of-life issues have become an increasingly interesting topic of discussion for both governments, businesses and societies around the world. In recent years, it has been regarded one of the great environmental liabilities and economic opportunities. One of the newest topics within sustainable business practices has been the adaptation of circular supply chains.
For a company to transition from a linear supply chain to a circular one, changes of business models are required. It requires a paradigm shift in the way goods are designed, manufactured, and consumed, and a closed-loop philosophy at the heart of each business case. Hence, a company must not only create new business processes but also innovate existing ones with emphasis on maintaining control of the products and materials throughout their entire life cycle. In the light of their Circular for Zero strategy, Novo Nordisk have tried to accommodate such innovation through the creation of the ReturPen™ pilot project. A project that aims to make Novo Nordisk truly sustainable by taking ownership of the end-of-life related waste of their insulin pens. [1] The vision of the pilot project is to create both environmental and social value, while reducing costs simultaneously. However, is this possible?
Introduction of Novo Nordisk
Novo Nordisk A/S is a multinational pharmaceutical company headquartered in Bagsværd, Denmark, with production facilities in more than 8 countries. They are Denmark’s largest company in terms of market value. Novo Nordisk employs more than 45,000 people across 76 countries and markets their products in more than 176 countries – the United States and China being their largest markets. Novo Nordisk is currently the market leader within the diabetes care market sitting on a market share of 45%. The company was created in 1989 through the merger of two Danish companies that date back to the 1920s. They manufacture and market pharmaceutical products and services specifically diabetes care medications and devices. However, they are also involved with hemostatis management, growth hormone therapy, and hormone replacement therapy. In 2019 they were ranked the world’s sixth biggest pharmaceutical company measured by market value. At the time of measuring, Novo Nordisk had a market value of USD 117.3 billion (DKK 797.3 billion), making them the most valuable company in Scandinavia.[2]
The Pharma Supply Chain Problem

Figure 1. The current linear supply chain in the pharmaceutical industry
The pharmaceutical industry contributes to health care systems and as such can be expected to contribute to finding new ways of limiting environmental impact. Figure 1 illustrates how the pharmaceutical industry has an obligation to do more. As one of the largest pharmaceutical companies fighting diabetes, Novo Nordisk has taken on this responsibility. Novo Nordisk produce and distributes 550 million insulin pens annually, with each device consisting of approximately 77 percent plastic, the rest being metal and glass. Over the course of 10 years, billions of pens and vials have ended up as landfill. As each pen or vial takes over 100 years to decompose, its product is an environmental sinner.[3] As part of its Circular for Zero strategy Novo Nordisk has created The ReturPen™ pilot project which aims to achieve the mission of a circular product-life cycle.
The ReturPen™ Pilot Project
On December 1st, 2020, Novo Nordisk launched its ReturPen™ Pilot Project to collect used insulin pens in the three selected municipalities of Copenhagen, Kolding and Aarhus. The project is in collaboration with 15 selected partners and will run until the end of 2021. The idea is that the insulin users return their used insulin pens at their local pharmacy, whereas Novo Nordisk will collect and recycle them into new raw materials. The aim of the pilot project is for Novo Nordisk to scale it nationally to all of Denmark and subsequently to other countries. The project is based upon existing distribution networks, allowing for low costs and relatively low risk.
The idea of the project is to close the loop in the supply chain of insulin pens and reduce clinical waste. The project meets the triple bottom line performance criteria as it fulfils all three measures of economic, social, and environmental responsibility. It is environmentally responsible as it recycles plastic, glass and metal while reducing carbon emission. At the same time, the project is socially responsible since Novo Nordisk has found a solution to the waste it creates as a company. Lastly, the project is economically responsible since the project is financially feasible for Novo Nordisk. The project, therefore, meets the triple bottom line expectations as it establishes the practice of a circular supply chain for insulin pens.
The ReturPen™ Partners
The ReturPen™ take-back system aims to mobilize industry collaboration to set new standards and guide users to recycle through a pharmacy drop-off solution. To achieve such an ambitious goal, Novo Nordisk has established a partnership with fifteen organizations covering the entire value chain. As figure 3 illustrates, each company is a key player within their area of expertise and have been meticulously selected to provide key insights into potential obstacles and opportunities. As the importance of Novo Nordisk, the initiator, has already been addressed, their function will not be described below.

Figure 2. The ReturPen™ partners
The Danish Environmental Protection Agency (EPA) is a part of the Ministry of Environment and Food and is the national authority on environmental and nature protection in Denmark. They play a vital role in the partnership as they provided the final dispensation for Novo Nordisk to establish the take-back program and ensure compliance with national legislation. Additionally, the municipalities of Copenhagen, Aarhus and Kolding ensure local compliance as laws, such as waste management, can vary locally. The Danish Association of the Pharmaceutical Industry (LIF) aims to provide the best possible conditions for the industry through lobbying. Hence, they attempt to influence legislators and the decisions of the government.
One of the most important links within the take-back supply chain is the logistics and operations providers. When establishing the partnership, Novo Nordisk partnered up with Nomeco, Tjellesen Max Jenne (TMJ), PostNord A/S and DHL.
Nomeco operates more than 300 pharmacy storage facilities across Denmark and acts as the market leader with 66 percent of the Danish pharmaceutical logistics market. They are a part of the Phoenix Group, the second-largest healthcare provider in Europe with 153 distribution centres across Germany and an annual revenue of €27.3 billion in 2020.[4] TMJ, another pharmaceutical wholesaler and distributor, is the second-largest company within the Danish pharmaceutical logistics market. [5] They cater for the remaining 33% of the Danish market and are a part of the American McKessen Corporation. The McKessen Corporation is a global pharmaceutical distributor with an annual revenue of $231.051 billion, making them the 8th highest-ranked American corporation on the Fortune 500.[6] PostNord A/S is the Nordic market leader within transport, distribution and logistics. Owned by the Swedish and Danish government, the company handle 500.000 parcels and transport 60.000 pallets of goods every day, resulting in annual revenues of €33.6 billion in 2020.[7] Lastly, we have DHL, the world’s largest courier, package delivery and logistics provider. The company delivers more than 1.5 billion parcels per year and achieved revenues of €66,8 billion in 2020.[8] In our analysis, to represent the logistics and operation providers in the ReturPen™ take-back system, an interview was conducted with the Operations Manager of Nomeco and the Chief Executive Officer of TMJ.
The partnership also includes four organizations that represent people with diabetes. As the users are the one’s handing in the pens, they play a vital role in the success of the project. Without the interest and convenience of the users, there will be no pens to collect. Steno Diabetes Center Copenhagen and Steno Diabetes Center Aarhus are a collaboration between the public and private sector. They are a hospital and ambulatory specialized in diabetes treatment, research and prevention. The Danish Diabetes Association act as an interest organization, and with more than 90,000 members, they represent roughly a third of all type-1 and type-2 diabetics in Denmark.[9] TYPE1 is an independent policy institute aiming to ensure sustainable diabetes treatment for all Danish type-1 patients. Together these four organizations provide a wholesome insight into the user aspect. The Steno Diabetes Centers provide technical know-how, the Diabetes Association provide user insights and the most substantial user reach, and TYPE1 provide strong communicational skills and insight into user behavior. In our analysis, to represent the user part of the supply chain, interviews have been conducted with the Deputy Chairman of the Executive Board of the Diabetes Association, the founder and chairman of TYPE1, and the Chief Executive Officer.
Lastly, we have the pharmacies that act as the drop-off location for returned pens. The Danish Pharmacy Association consists of 196 members, which employ 5.600 pharmacists. As a trade association, they represent the interest of the pharmacies through national and international collaborations and partnerships.[10] To represent the pharmacy link of the supply chain, an interview has been conducted with the Director of Health of the Danish Pharmacy Association.
Is the ReturPen™ project a sustainable solution?
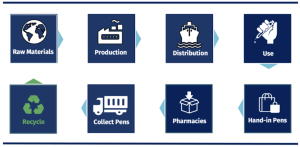
Figure 3. Overview of the circular supply chain of the ReturPen™ take-back system
From a triple bottom line perspective Novo Nordisk’s ReturPen™ Pilot Project was very successful as seen in figure 4. The pilot project has shown a high degree of social responsibility. Novo Nordisk believe this issue must be solved together with industry partners and as a result, the ReturPen™ project enjoys a high degree of industry collaboration. Novo Nordisk has obtained a high degree of industry support from its fifteen partners, which has attracted significant attention from the Danish Environmental Protection Agency6. In Denmark, all waste management is adapted to the local legislations of municipalities, although the procedures must comply with the national regulations of Denmark and the European Union. When establishing the ReturPen™ take-back system, Novo Nordisk had to receive permission to handle the clinical waste. The Minister of the Environment, Lea Wermelin, allowed temporary permission to handle clinical waste as 63% of all plastic currently is incinerated in Denmark.[11] She gave this permission with the intention of Novo Nordisk turning the plastic waste into new resources. As the consumers are very important actors in this circular supply chain, Novo Nordisk has drawn much attention to consumer needs and closely collaborated with them to create a project that truly works for all stakeholders.
Additionally, the pilot project has proven a high degree of environmental responsibility through efficient waste management. Scholars such as Poritt[12] and Bansal[13] have labelled environmental responsibility as the most important of the three triple bottom line pillars, making social and economic secondary, as everything depends on the earth’s limitary resources. Through an efficient circular supply chain, Novo Nordisk can achieve a lower cost of production while simultaneously decreasing its environmental impact. The ReturPen™ take-back system allows for the reclamation of raw materials from existing goods, which can be re-used for manufacturing, saving money on materials, reducing risk from resource depletion, lowering carbon emissions, and ensuring regulatory compliance. Novo Nordisk has forecasted that the sales of single-use pens will increase over time whereas it never will be optimal if their products end up in a landfill. Therefore, implementing a circular supply chain provides the opportunity to tackle a problem that is here to stay. Novo Nordisk has focused on getting plastic out of incineration to reduce the CO2 emission and re-use the plastic. Additionally, the mission of the ReturPen™ system is to collect the used pens, recycle them, divide them into new reused glass, plastic and metal, which can then be given a new life.
Lastly, Novo Nordisk will see a growing degree of economic responsibility going forward. As with any other innovative project, the initiator must pay the price of being a first mover. However, as the take-back system use existing distribution channels to retrieve the handed-in pens, Novo Nordisk have found a way to combine the new business model with the old ones – allowing for a reduced cost of innovation. Nonetheless, the pilot project is still a proof of concept and will be developed and scaled nationally, and subsequently internationally. Therefore, project expansion and project cost will go hand-in-hand. Furthermore, this investment can be successful for Novo Nordisk’s long-term shareholder value as it is becoming increasingly expensive for a company not to be sustainable.
What does the future of pharma require?
Based on the analysis of the ReturPen™ Pilot Project we have outlined the following key learnings. These factors are vital if other companies want to establish a sustainable initiative similar to the one of Novo Nordisk.
Firstly, it is paramount to align the expectations and communication among all participating partners. The ReturPen™ project has been very successful in this regard and have achieved a high trust and strong collaboration among all 15 partners as they all have the same sustainability ambitions.
Secondly, it is important to align expectations among partners of what will happen with the waste that has been collected. As this is a pilot project, it will not be right the first time. It needs constant tweaking while new learnings occur. Moreover, as the project is the first of its kind, technologies that will turn used pens into new pens has not been developed yet. As a result, it is important to be creative until such a solution is found. Novo Nordisk have been very successful at this. They found a solution to overcome this temporary barrier, where they turned the collected pens into chairs, bags, cups and lamps. Even though this is not the ambition of the project, it has been a great initiative providing old products new life, while waiting for a more scalable final destination.
Lastly, it is crucial to keep the users involved throughout the entire process as the success of the project depends greatly on them. No handed-in pens equals no recyclable materials. Novo Nordisk have been very successful at establishing strong collaboration using user-feedback and user representatives through their partner structure. Both parts have responded with the same enthusiasm and shown great excitement regarding the project. As many users are highly conscious of the waste their illness creates, the project has provided a unique opportunity to be a part of the next life cycle of their used insulin pen.
All of the above mentioned are important factors to consider, when trying to establish an ahead of the game sustainability project. Through the ReturPen™ project, Novo Nordisk have shown what is required to create a truly sustainable supply chain initiative, which will be even more successful when scaled nationally in 2022.
Is this the future of pharma?
Based on the key learning of the ReturPen™ pilot project, Novo Nordisk aims to scale the project. As this initiative is not a one size fits all, it will be crucial to consider local differences when adapting the project. The mission of the project is to support Novo Nordisk’s strategy “Circular for Zero”, which aims to adapt new environmental strategies with the aspiration to have zero environmental impact. Even though the project does not remove the environmental impact just yet, it is safe to say that it is definitely an important step in the right direction. A step that, hopefully, will inspire other pharmaceutical companies to do the same.
 Forfatter: Christine Klara Rohde
Forfatter: Christine Klara Rohde
• Name: Christine Klara Rohde
• Title: Student Assistant at A.P. Moller – Maersk
• Studies: MSc. Finance and Strategic Management
• E-mail: ChristineKlaraRohde@gmail.com
• LinkedIn: Linkedin.com/in/christinerohde
 Forfatter: Alexander Ahlmann Pedersen
Forfatter: Alexander Ahlmann Pedersen
• Name: Alexander Ahlmann Pedersen
• Title: Student Assistant at Novo Nordisk
• Studies: MSc. Finance and Strategic Management
• E-mail: AlexanderAhlmann@outlook.com
• LinkedIn: Linkedin.com/in/alexanderahlmann
Christine and Alexander met in 2018 at Copenhagen Business School where they both started their bachelor’s degree in International Shipping and
Trade. During their second year, they were two of the students selected for the tri-continental elite programme, Global SCLM (Supply Chain and
Logistics Management). A program consisting of one semester at each of the three partnering universities: Copenhagen Business School in Denmark,
University of British Columbia in Canada and the Chinese University of Hong Kong in Shenzhen, China. Unfortunately, due to COVID-19 their semester
in Canada was cut short, while their semester in China was conducted online. It was during this time, Alexander started working at Novo Nordisk as an
intern in supply chain planning, while Christine worked at Female Invest. Today, Alexander still works at Novo Nordisk as a Student Assistant, whereas
Christine works at Maersk in the decarbonization department. Additionally, they both started their master’s in Finance and Strategic Management at
Copenhagen Business School in September 2021.
References
Novo Nordisk. (2020). ReturPen™ . https://www.ReturPen™ .dk/
Novo Nordisk. (2020). Annual Report. Novo Nordisk Annual Report 2020 (novonordisk.com)
Novo Nordisk (2020). Circular for Zero. https://www.novonordisk.com/sustainable-business/zero-environmental-impact.html
Nomeco. (2020). Annual Report. https://nomecotest.dk/en/nomecos-annual-report-growth-decline- earnings
Phoenix. (2020). Annual Report. https://www.thephoenixgroup.com/investor-relations/results-
centre/2020.aspx
Tjellesen Max Jenne A/S. (2020). Annual Report.
https://regnskaber.cvrapi.dk/21819097/amNsb3VkczovLzAzL2VjLzdhL2VhLzJkL2QzOT AtNG
NjNi1iMTMxL TFlOGU3ZWJkYmQ3OA.pdf
McKesson. (2020). Annual Report – Improving healthcare in every setting.
Postnord (2020) Annual Report. https://www.postnord.dk/siteassets/pdf/finansiell-information/arsregnskabsmeddelser/aarsrapport_2020.pdf
DHL. (2020). Annual Report. https://annualreport2017.dpdhl.com/downloads- ext/en/documents/DPDHL_2017_Annual_Report.pdf
SDCC. (2020). Steno Diabetes Center Copenhagen.
Danmarks Apotekerforening (2020). Annual Report. https://www.apotekerforeningen.dk/-/media/apotekerforeningen/aarbog-laegemidler-i-danmark-2020-21.pdf?la=da&hash=75F5375D810A6CABB4E8201D2226B1EF326FE9F5
The Danish Government (2018). Plastics without waste – The Danish government’s plastics action plan. https://en.mfvm.dk/fileadmin/user_upload/ENGLISH_FVM.DK/Regeringens_plastikhandlingsplan_UK.pdf
Porritt, J. (2007). Sustainability for All. https://www.youtube.com/watch?v=39bPjnFBt-o
Bansal, T. (2005). Evolving sustainably: A longitudinal study of corporate sustainable development. Strategic Management Journal, 26, 197–218. https://doi.org/10.1002/smj.441
[1] Novo Nordisk. (2020c). ReturPen™ . https://www.ReturPen™ .dk/
[2] Novo Nordisk. (2020). Annual Report. Novo Nordisk Annual Report 2020 (novonordisk.com)
[3] Novo Nordisk (2020). Circular for Zero. https://www.novonordisk.com/sustainable-business/zero-environmental-impact.html
[4] Nomeco. (2020). Annual Report. https://nomecotest.dk/en/nomecos-annual-report-growth-decline- earnings
[5] Tjellesen Max Jenne A/S. (2020). Annual Report. https://regnskaber.cvrapi.dk/21819097/amNsb3VkczovLzAzL2VjLzdhL2VhLzJkL2QzOT AtNG NjNi1iMTMxL TFlOGU3ZWJkYmQ3OA.pdf
[6] McKesson. (2020). Annual Report – Improving healthcare in every setting.
[7] Postnord (2020) Annual Report. https://www.postnord.dk/siteassets/pdf/finansiell-information/arsregnskabsmeddelser/aarsrapport_2020.pdf
[8] DHL. (2020). Annual Report. https://annualreport2017.dpdhl.com/downloads- ext/en/documents/DPDHL_2017_Annual_Report.pdf
[9] SDCC. (2020). Steno Diabetes Center Copenhagen.
[10] Danmarks Apotekerforening (2020). Annual Report. https://www.apotekerforeningen.dk/-/media/apotekerforeningen/aarbog-laegemidler-i-danmark-2020-21.pdf?la=da&hash=75F5375D810A6CABB4E8201D2226B1EF326FE9F5
[11] The Danish Government (2018). Plastics without waste – The Danish government’s plastics action plan. https://en.mfvm.dk/fileadmin/user_upload/ENGLISH_FVM.DK/Regeringens_plastikhandlingsplan_UK.pdf
[12] Porritt, J. (2007). Sustainability for All. https://www.youtube.com/watch?v=39bPjnFBt-o
[13] Bansal, T. (2005). Evolving sustainably: A longitudinal study of corporate sustainable development. Strategic Management Journal, 26, 197–218. https://doi.org/10.1002/smj.441